2022-06-06 来源 : 医药笔记 ,作者唐钧
2022年6月5日,复宏汉霖PD-1抗体斯鲁利单抗一线治疗广泛期小细胞肺癌(ES-SCLC)的三期临床数据由吉林省肿瘤医院程颖教授在ASCO会议上做口头汇报。
尽管有2款PD-L1抗体获批治疗ES-SCLC,预后仍然极差
世界卫生组织(WHO)估计2020年全球有约220万肺癌新发病例,其中15%即33万病例为小细胞肺癌。ES-SCLC约占所有SCLC的三分之二,且预后极差,五年生存率仅7%。与以往化疗标准疗法相比,免疫检验点如PD-L1抗体+化疗联合治疗可以带来25%的生存改善。FDA和EMA已经批准罗氏Atezolizumab、阿斯利康Durvalumab联合化疗治疗ES-SCLC,美国国立癌症综合网络(NCCN)也将这两种治疗方案列为首选方案。
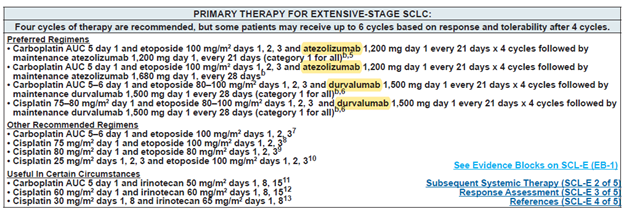
图1 NCCN 指南中ES-SCLC的一线治疗方案
但是,IMpower133临床中Atezolizumab+化疗联合治疗仅能延长2个月的mOS,CASPIAN临床中Durvalumab+化疗联合治疗仅能延长2.7个月的mOS。此外,目前还没有PD-1抗体或PD-1+化疗被证实可以带来生存改善。因此,临床上亟需可以显著改善ES-SCLC患者生存的、更有效的免疫检验点疗法。
ASTRUM005:斯鲁利单抗治疗ES-SCLC的三期临床达到OS主要终点和所有次要终点

斯鲁利单抗为一款PD-1抗体,在多个瘤种中表现出临床获益。由于斯鲁利单抗在二期临床ASTRUM010中表现出OS主要终点的改善,以及更好的安全性数据,斯鲁利单抗于2022年3月获得NMPA批准上市,用于治疗晚期不可切除或转移性的MSI-H实体瘤。
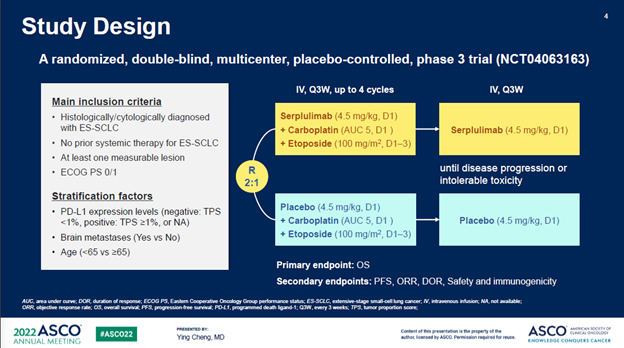

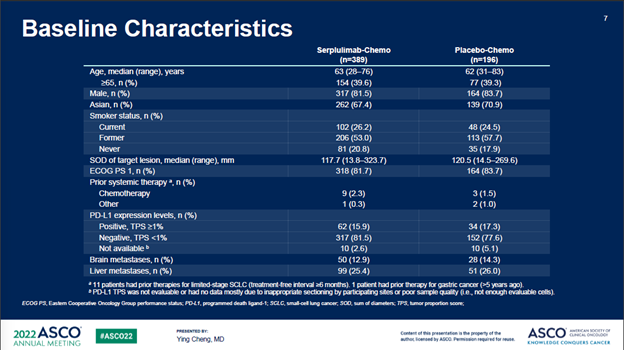
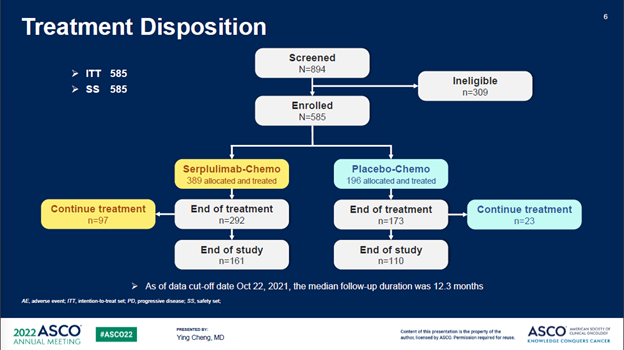
ASTRUM005在6个国家的114个中心入组了585例患者,按照2:1的比例随机分组。研究的主要终点为OS,次要终点包括PFS、ORR、DOR、安全性和免疫原性。585例入组患者中,389例随机分配到斯鲁利单抗+化疗联合治疗组,196例随机分配到安慰剂+化疗对照组。两组很好的平衡了基线特征。

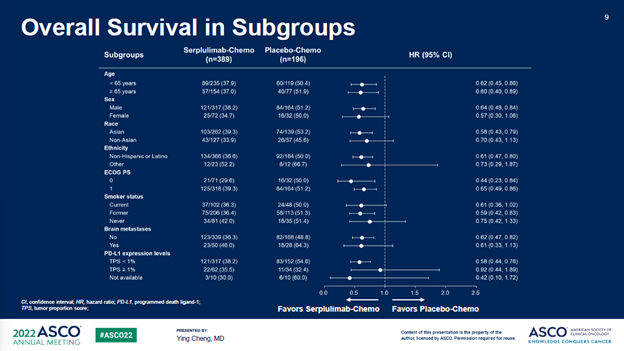
截至2021年10月22日,经过平均12.3个月的随访时间,斯鲁利单抗+化疗组的mOS为15.4个月,安慰剂+化疗组为10.9个月,治疗组带来的4.5个月的mOS改善,死亡风险降低37%(P<0.001)。重要的是,亚组分析中亚裔人群和非亚裔人群没有显著差异(HR分别为0.61和0.73)。
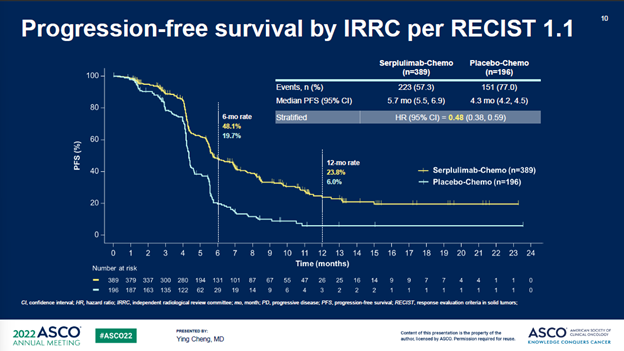

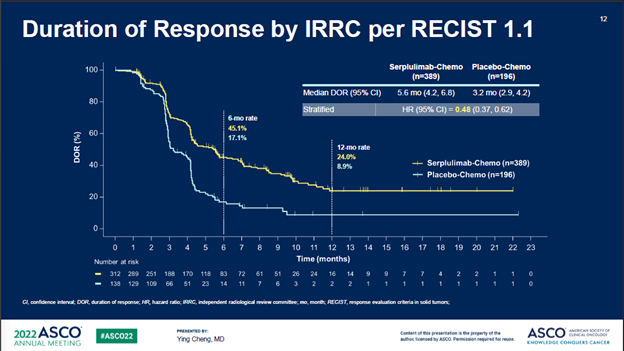
此外,斯鲁利单抗+化疗组相比于安慰剂+化疗组,所有次要终点都达到显著改善,进一步支持了具有临床意义的OS获益。


对于3级及以上治疗相关副作用发生率,斯鲁利单抗+化疗组为33.2%,安慰剂+化疗组为27.6%。

综上所述,斯鲁利单抗+化疗为ES-SCLC患者带来显著生存获益,死亡风险降低37%,同时所有次要终点达到显著改善。此外,实验组和对照组的安全性具有可比性,表现出可控的安全性。
ASTRUM005与其他ES-SCLC 关键临床数据对比
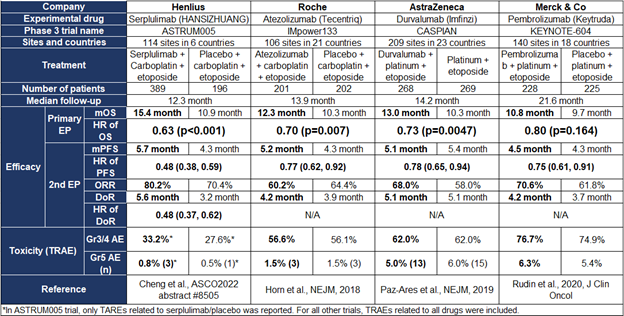
对比ASTRUM005与其他三项ES-SCLC临床IMpower133、CASPIAN、KEYNOTE-604,HR分别为0.63、0.70、0.73和0.80,ASTRUM005死亡风险的降低幅度最大。此外,ASTRUM005的所有次要终点包括PFS、ORR、DOR都达到统计学显著改善,而其他3项临床都没有达到这些次要终点的统计学显著改善。ASTRUM005临床中4.5个月的mOS延长,也是4项临床研究中最长的。
表1. ES-SCLC四项关键三期临床数据对比
斯鲁利单抗联合治疗ES-SCLC的监管路径
斯鲁利单抗+化疗联合治疗证实了相比于化疗的临床获益,和其他两款PD-L1抗体具有可比性(如果不是更好的话)。斯鲁利单抗是首个一线治疗ES-SCLC优于化疗的PD-1抗体。复宏汉霖已经向NMPA递交上市申请,并计划在今年下半年向EMA递交上市申请。斯鲁利单抗于2022年4月获得FDA的孤儿药认定。复宏汉霖预期斯鲁利单抗将从ES-SCLC开始获得多项监管批准。我们感谢ASTRUM005临床研究中做出巨大贡献的所有患者和研究者。我们坚信斯鲁利单抗如果被纳入治疗选择,将会改变ES-SCLC的治疗格局。
以下为英文版:
Henlius’ serplulimab ASTRUM005 international pivotal trial showed remarkable primary endpoint of overall survival benefits in the first-line treatment setting for extensive-stage small cell lung cancer
Jun (Leon) Tang*1,, PhD; Van Chau1,, PharmD; QingyuWang1,, MD.
1 Shanghai Henlius Biotechnology
9F, Innov Tower, 1801 Hongmei Road, Shanghai, 200233
Email: jtang@henlius.com
*WeChat account: junt806
Despite 2 anti-PD-L1 drugs approved to treat extensive stage small-cell lung cancer, prognosis remains dire
World Health Organization (WHO) estimates approximately 2.2 million new cases of lung cancer in 2020 globally, of which about 15% are estimated to be small cell lung cancer (SCLC) cases, total ~330,000 new SCLC cases (https://www.iarc.fr/). Extensive stage of SCLC (ES-SCLC) accounts for 2/3 of all SCLC cases, and ES-SCLC has a very poor prognosis, with less than 7% patients who can survive 5 years. Immune checkpoint molecules such as PD-L1 blocking monoclonal antibodies (mAbs)/chemo combo showed ~25% overall survival benefits compared to the previous chemotherapy standard of care (SoC). The two PD-L1 mAbs, namely atezolizumab and durvalumab, in combo with chemo were approved by the FDA and the EMA to treat ES-SCLC, and both therapies are listed as the preferred regimens in the national comprehensive cancer network guidelines (NCCN) of the United States (Figure 1).
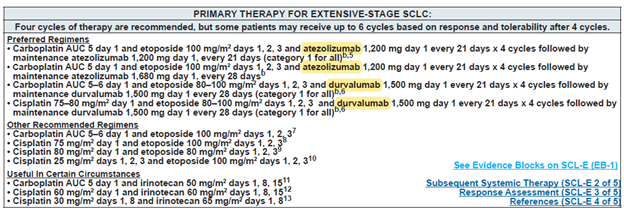
Figure 1. NCCN guidelines on the first line treatment for ES-SCLC.
However, the median overall survival (mOS) of atezolizumab/chemo combo in IMpower133 trial showed only a 2-month survival benefit, while mOS of durvalumab/chemo combo in CASPIAN trial only a 2.7-month. Furthermore, no anti-PD-1 mAb/chemo combo has shown significant improvement over chemotherapy in this setting. Therefore, more effective treatment options resulting significant OS benefit are urgently needed in the checkpoint inhibitor armamentarium to combat the aggressive ES-SCLC.
ASTRUM005 Phase 3 trial for ES-SCLC hit primary OS endpoint and all secondary endpoints

Serplulimab is an anti-PD1 mAb that has shown clinical benefits in multiple tumor types. Due to its significant improvement to the primary endpoint of overall response rate coupled with numerically better adverse drug events profile from ASTRUM010 Phase 2 clinical trial in tissue agnostic histologies, Serplulimab was approved by China’s NMPA for the treat of advanced unresectable or metatstatic MSI-H solid tumors in March 2022 [Qin, ASCO 2021 annual meeting].


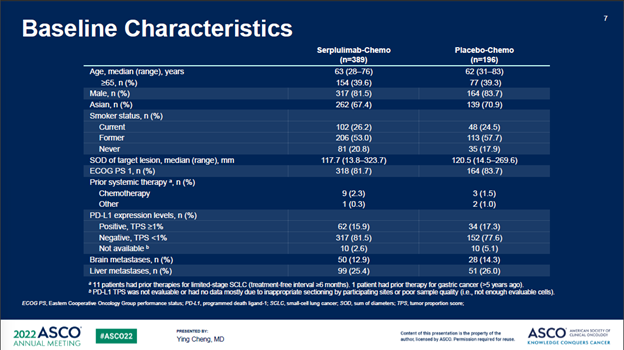

ASTRUM005 enrolled 585 patients from 114
sites in 6 countries
(China, Russia, Turkey, Poland, Georgia, and Ukraine), and the patients were randomized at a 2:1 ratio. The primary
endpoint was overall survival (OS), and secondary endpoints included PFS, ORR,
DOR, safety, and immunogenicity. Among the 585 enrolled patients, 389 were randomized to the
serplulimab/chemo arm while 196 to the placebo/chemo control arm. Baseline
characteristics was well balanced between the two arms.


After a median follow-up of 12.3 months as of the cut-off date of Oct 22nd, 2021, the median OS (mOS) of the serplulimab/chemo arm was 15.4 months vs 10.9 months in the control arm, a remarkable 4.5-month additional survival benefits resulting in an OS hazard ratio (HR) of 0.63 (P < 0.001). Importantly, subset analysis showed no statistically significant difference between the Asian and Non-Asian patients (HR of 0.61 vs 0.73).

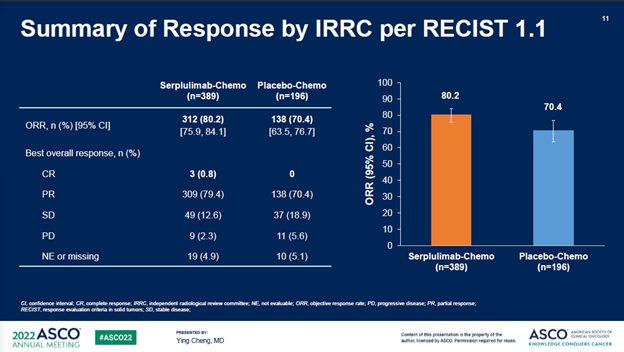

In addition, all secondary endpoints including PFS, ORR, and DOR showed statistically significant improvement in the serplulimab/chemo arm vs the placebo/chemotherapy control, further supporting the clinically meaningful OS benefits.

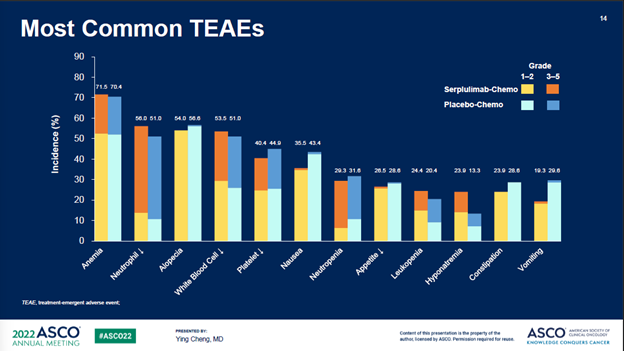
Grade 3 and above treatment related adverse events (TRAEs) related to serplulimab/placebo occurred in 33.2% in serplulimab/chemo arm vs 27.6% in chemo control arm.

In conclusion, serplulimab/chemo combo showed clinical meaningful survival benefit in ES-SCLC, evidenced by the primary endpoint OS HR of 0.63, as well as significant improvements in all secondary efficacy endpoints. In addition, the toxicity was comparable between experimental and control arm, showing a manageable safety profile.
ASTRUM005 compares favorably with other ES-SCLC pivotal trials
Comparing ASTRUM005 with 3 other Phase 3 pivotal trials, namely IMpower133, CASPIAN, and KEYNOTE-604, the primary endpoint of OS HR of ASTRUM005 was 0.63, versus 0.70 from IMpower133, 0.73 from CASPIAN, and 0.80 (not statistically significant) from KEYNOTE-604. In addition, all secondary endpoints of ASTRUM005 (namely PFS, ORR, and DOR) show statistically significant and clinically meaningful improvement over control, but none of the other 3 pivotal trials showed statistically better benefits in all the 3 secondary endpoints. Most importantly, a 4.5-month overall survival benefit with Serplulimab is numerically the largest amongst all 4 pivotal trials (Table 1).
Table 1. Efficacy and safety comparison amongst the 4 Ph3 pivotal trials for ES-SCLC.

The regulatory path for serplulimab combo in ES-SCLC
Serplulimab/chemo combo demonstrated clinical meaningful benefits vs chemotherapy, as well as comparable benefits with (if not numerically better than) the current two anti-PD-L1 mAb SoC. Serplulimab is the first anti-PD-1 mAb to show statistically significant and clinical meaningful improvement vs chemotherapy in ES-SCLC first line setting. Henlius has submitted the data package to China’s NMPA for ES-SCLC and plans to submit to the EMA in 2H2022. Serplulimab received orphan drug designation from the US FDA in April 2022. Henlius expects multiple regulatory approvals of this life-saving therapy starting with the most aggressive lung cancer, ES-SCLC. We are very grateful of all the patients in our clinical trials and the investigators who made tremendous contributions to ASTRUM005. We are confident that having serplulimab included in the treatment option would shift the therapy paradigm for ES-SCLC.
References:
1.Cheng et al., ASTRUM005: serplulimab, a novel anti-PD-1 antibody plus chemotherapy versus chemotherapy as first-line treatment for extensive-stage small-cell lung cancer: an international randomized phase study. ASCO annual meeting 2022
2.World Health Organization Global Cancer Observatory (https://www.iarc.fr/)
3.National Comprehensive Cancer Network Guidelines (www.nccn.org)
4.Horn et al., first-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. NEJM, 2018.
5.Paz-Ares et al., Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomized, controlled, open-label, phase 3 trial. Lancet, 2019.
6.Rudin et al., Pembrolizumab or placebo plus etoposide and platinum as first-line therapy for extensive-stage small-cell lung cancer: randomized, double-blin, phase III KEYNOTE-604 study. J Clin Oncol, 2020.
7.Qin et al., Efficacy and safety of HLX10, a novel anti-PD-1 antibody, in patients with previously treated unresectable or metastatic microsatellite instability-high or mismatch repair-deficient solid tumors: A single-arm, multicenter, phase 2 study. ASCO 2021 annual meeting.
----------------------
版权声明:本网站所有注明来源“医微客”的文字、图片和音视频资料,版权均属于医微客所有,非经授权,任何媒体、网站或个人不得转载,授权转载时须注明来源:”医微客”。本网所有转载文章系出于传递更多信息之目的,且明确注明来源和作者,转载仅作观点分享,版权归原作者所有。不希望被转载的媒体或个人可与我们联系,我们将立即进行删除处理。 本站拥有对此声明的最终解释权。




发表评论
注册或登后即可发表评论
登录注册
全部评论(0)