2020-03-16


观察性研究:有长有短
 图片来源:参考资料[2]
图片来源:参考资料[2]


随机化试验:魔力不减

解决方案:因势利导
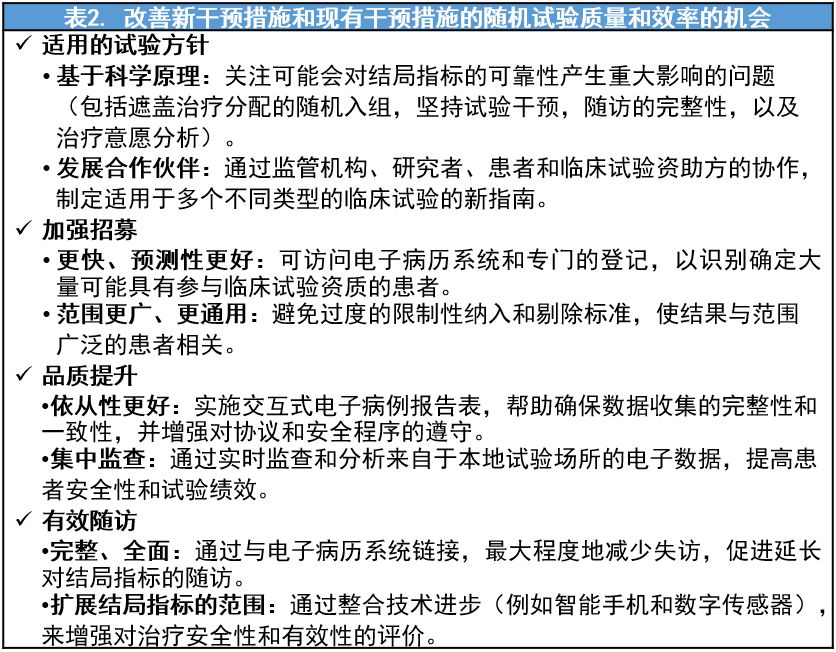

参考文献:
[1] Collins R, Bowman L, Landray M, et al. The Magic of Randomization versus the Myth of Real-World Evidence. N Engl J Med. 2020 Feb 13; 382(7):674-678. doi: 10.1056/NEJMsb1901642
[2] Galson, S., and G. Simon. Real-world evidence to guide approval and use of new treatments. Discussion Paper, National Academy of Medicine, Washington, DC. Oct 18, 2016. Retrieved Feb 17, 2020 from https://nam.edu/wp-content/uploads/2016/10/Real-World-Evidence-to-Guide-the-Approval-andUse-of-New-Treatments.pdf.
[3] National Academies of Sciences, Engineering, and Medicine. Examining the impact of real-world evidence on medical product development: proceedings of a workshop series. Washington, DC: National Academy of Sciences, 2019. Retrieved Feb 17, 2020 from http://nationalacademies.org/HMD/Reports/2019/examining -impact-real-world-evidence-on-medical-product-development-proceedings.aspx).
[4] Paul Glasziou, Iain Chalmers, Michael Rawlins, et al. When are randomised trials unnecessary? Picking signal from noise. BMJ. 2007 Feb 17; 334(7589): 349–351. doi: 10.1136/bmj.39070.527986.68
[5] Nielsen SF, Nordestgaard BG, Bojesen SE. Statin use and reduced cancer-related mortality. N Engl J Med 2012; 367: 1792-802. doi: 10.1056/NEJMc1214827.
[6] Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet 2016; 388:2 532-61. doi: 10.1016/S0140-6736(16)31357-5
[7] Roberts I, Prieto-Merino D. Applying results from clinical trials: tranexamic acid in trauma patients. J Intensive Care 2014; 2:5 6. doi: 10.1186/s40560-014-0056-1
[8] Eapen ZJ, Lauer MS, Temple RJ. The imperative of overcoming barriers to the conduct of large, simple trials. JAMA 2014; 311: 1397-8. doi: 10.1001/jama.2014.1030
[9] Reith C, Landray M, Devereaux PJ, et al. Randomized clinical trials — removing unnecessary obstacles. N Engl J Med 2013; 369:1 061-5. doi: 10.1056/NEJMsb1300760
[10] Stewart DJ, Whitney SN, Kurzrock R. Equipoise lost: ethics, costs, and the regulation of cancer clinical research. J Clin Oncol 2010; 28: 2925-35. doi: 10.1200/JCO.2009.27.5404
[11] Perkovic V, Jardine MJ. Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019; 380: 2295-306. doi: 10.1056/NEJMoa1811744
[12] Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002; 360: 7-22. DOI:10.1016/S0140-6736(02)09327-3
[13] Lindblad AS, Manukyan Z, Purohit-Sheth T, et al. Central site monitoring: results from a test of accuracy in identifying trials and sites failing Food and Drug Administration inspection. Clin Trials 2014; 11:2 05-17. doi: 10.1177/1740774513508028
[14] Pogue J, Walter SD, Yusuf S. Evaluating the benefit of event adjudication of cardiovascular outcomes in large simple RCTs. Clin Trials 2009; 6:2 39-51. doi: 10.1177/1740774509105223
[15] Ndounga Diakou LA, Trinquart L, Hróbjartsson A, et al. Comparison of central adjudication of outcomes and onsite outcome assessment on treatment effect estimates. Cochrane Database Syst Rev 2016; 3: MR000043
[16] Kahan BC, Feagan B, Jairath V. A comparison of approaches for adjudicating outcomes in clinical trials. Trials. 2017 Jun 8;18(1):266. doi: 10.1186/s13063-017-1995-3
[17] Hlatky MA, Ray RM, Burwen DR, et al. Use of Medicare data to identify coronary heart disease outcomes in the Women’s Health Initiative. Circ Cardiovasc Qual Outcomes 2014;7 : 157-62. doi: 10.1161/CIRCOUTCOMES.113.000373
[18] Ford I, Murray H, McCowan C, Packard CJ. Long-term safety and efficacy of lowering low-density lipoprotein cholesterol with statin therapy: 20-year follow-up of West of Scotland Coronary Prevention Study. Circulation 2016; 133: 1073-80. doi: 10.1161/CIRCULATIONAHA.115.019014
[19] Herrington WG, Goldsack JC, Landray MJ. Increasing the use of mobile technology-derived endpoints in clinical trials. Clin Trials 2018; 15: 313-5. doi: 10.1177/1740774518755393
[20] Landray MJ, Bax JJ, Alliot L, et al. Improving public health by improving clinical trial guidelines and their application. Eur Heart J 2017; 38: 1632-7. doi: 10.1093/eurheartj/ehx086
[21] 药明康德. 权威报告:运用基于风险的监查, 让临床试验质量审核更高效. Nov 19, 2019. Retrieved Feb 17, 2020 from https://med.sina.cn/article_detail_103_2_74265.html
[22] Zachary Brennan. Hahn Stresses Importance of Data, RWE in First All-Hands Meeting. Jan 30, 2020. Retrieved Jan 31, 2020 from https://www.raps.org/news-and-articles/news-articles/2020/1/hahn-stresses-importance-of-data-rwe-in-first-all
百度浏览 来源 : 药明康德 药明康德
版权声明:本网站所有注明来源“医微客”的文字、图片和音视频资料,版权均属于医微客所有,非经授权,任何媒体、网站或个人不得转载,授权转载时须注明来源:”医微客”。本网所有转载文章系出于传递更多信息之目的,且明确注明来源和作者,转载仅作观点分享,版权归原作者所有。不希望被转载的媒体或个人可与我们联系,我们将立即进行删除处理。 本站拥有对此声明的最终解释权。




发表评论
注册或登后即可发表评论
登录注册
全部评论(0)